Welcome to 2022 User Group Meeting
AusDiagnostics is a leading Australian in vitro diagnostics company that specialises in the design and manufacture of molecular diagnostics products and automation technology.
Our clinical diagnostic solutions are used by laboratories and hospitals around the world to detect and test for a broad range of pathogens and conditions in humans, animals, and the environment.
Explore our latest high throughput solution, the Ultraplex 3 below or register for a live demonstration.
ExploreHighplex Alliance™
High-multiplexing for medium throughput
24 samples per run
Ultraplex Alliance™
High-multiplexing for high throughput
96 samples per run
Virtual Lab
Explore the Ultraplex 3
Book a Live Demo

Revolutionise your workflow
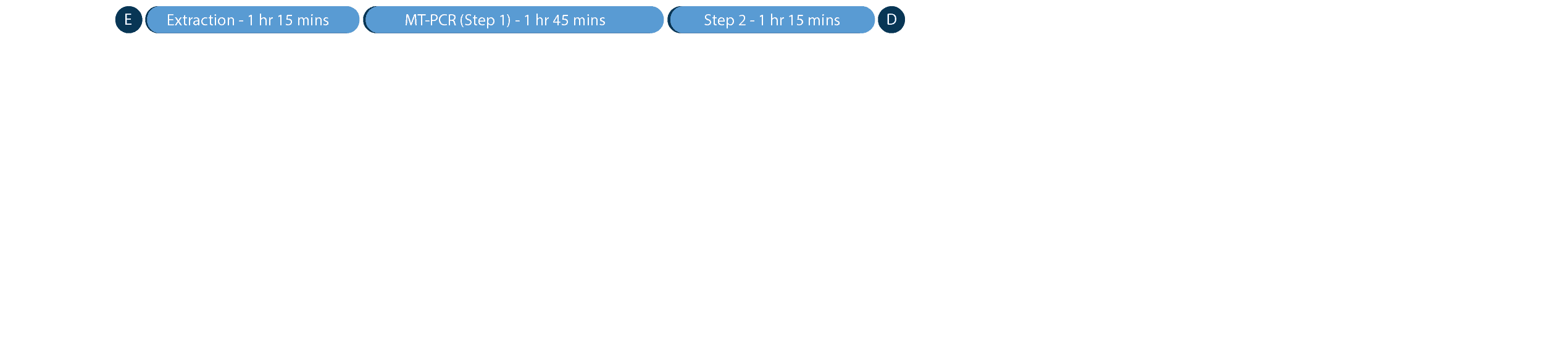
Combine the Ultraplex 3 with our MT-Prep™ XL’s DNA extraction capabilities to achieve testing for up to 960 samples per 24 hours.*
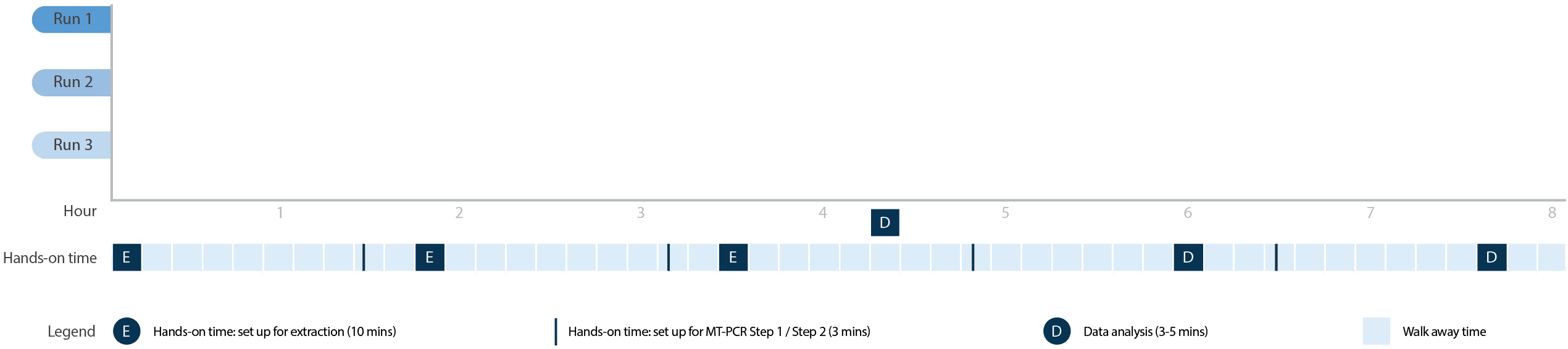
*Based on the extraction of 96 samples using the MT-Prep™ 96 extraction kit on the MT-Prep™ XL followed by MT-PCR on the Ultraplex 3 using 8-well universal panel.
Brochures
TandemPlex® (MT-PCR) Panel Brochures
| Critical Testing & Resistance | Enteric Pathogens |
| Genital Infections & STI | Respiratory Infections |
| Urinary Tract Infections | Dermatophytes & Other Fungi |
| Haemochromatosis |
Sample Purification – MT-Prep™ & Puryx®
| Extraction Portfolio | MT-Prep™ 24 |
| Puryx® Comprehensive Extraction | Puryx® Rapid Extraction |
| Puryx® Access Extraction |
Instrument Brochures
| Highplex | Highplex Alliance™ |
| Ultraplex 3 | Ultraplex Alliance™ |